Synthesis of hydrogen peroxide via antraquinone
Summary of the work made
We have studied, for many years, all the steps of the all-TETRA process , we have studied and optimized in particular: (i) the reactor of tetra-hydroanthraquinone hydrogenation, (ii) the oxidizing reactor, (iii) the active quinones regeneration, (iv) the start-up procedure for a new industrial plant. Our studies have been made following the mentioned reactors and operations respectively in: laboratory plants, pilot plants and industrial plants.
Work
Our group studied the know how of the hydrogen peroxide production for many years in collaboration with MONTEFLUOS SpA then AUSIMONT SpA (ex Montedison group) collaborating in increasing the production by eliminating the bottlenecks of the industrial plant of the Company that was located in Bussi (Pescara). Important results have been obtained by studying the process at different size levels, from laboratory scale, to pilot plant and industrial plant scale. All the production steps have been carefully studied and some of the collected information have been reported in different published works [1-9].
We have been involved also in defining the start up operation for a new plant by testing this operation in a laboratory continuous pilot plant which has been realized on purpose[8].
We have studied, in particular, the production of hydrogen peroxide via anthraquinone with the method named all-tetra. The process occurs in four steps, i.e.:
-
hydrogenation of 2-ethyltetrahydroanthraquinone (THEAQ) in a slurry reactor by using a Pd supported catalyst [1-5];
-
oxidation of the product obtained (THEAQH2) in a gas-liquid reactor [6];
-
extraction of hydrogen peroxide with an acid aqueous solution;
-
regeneration of active quinones from chemically deteriorated molecules and purification of the solution on a packed bed tubular reactor [7] and recycle;
In the mentioned process all-tetra, hydrogen peroxide is produced subjecting a mixture of 30% 2-ethylanthraquinone (EAQ) and 70% THEAQ dissolved in an appropriate mixture of solvents to cyclic reduction and oxidation as in the following scheme:
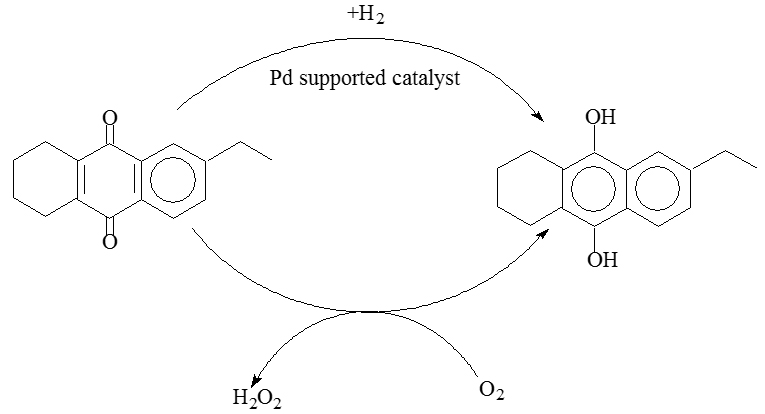
Hydrogenation is usually kept below 70% of conversion. In this way, only THEAQ is hydrogenated because of the presence of the following equilibrium

completely shifted to the right.
Hydrogenation on palladium is a very fast reaction occurring in few minutes in a laboratory semibatch reactor. Therefore, kinetic data are normally affected by internal diffusion limitation. For these reasons, kinetic runs can be performed only measuring the rates of hydrogen consumption for different catalyst hold-up and different stirring rates. The obtained results show an apparent zero order kinetics for hydrogen; from the shape of the curves of hydrogen consumption during time, declining at the end of the run, a first order kinetic law can be assumed for THEAQ. This behaviour suggests a Rideal-Eley mechanism for THEAQ hydrogenation:

The zero order observed for hydrogen can be explained considering the great affinity of hydrogen for palladium. It is reasonable to suppose that the dissociative adsorption equilibrium constant for hydrogen on palladium is very high. The kinetic behaviour of this hydrogenation can well be described by calculating an overall effectiveness factor considering all the mass transfer limitation steps. The reaction rate is, therefore:
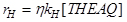
In all the runs examined, was always quite low (0.02-0.15) suggesting that only a thin shell of the catalyst particles is normally operative. In the industrial plant, the palladium-supported catalyst is normally subjected to progressive deactivation. As a consequence, fresh catalyst must be added during plant line operation to avoid a loss of productivity. We recognized two types of catalyst poisoning: a reversible one due to the adsorption of water on palladium catalytic sites, and an irreversible and much slower one probably due to the condensation of anthraquinone molecules with aromatic rings partially hydrogenated on the surface of the catalyst. As catalyst poisoning occurs very slowly, it must be studied in a continuous reactor. We created a continuous laboratory micropilot plant to study reversible poisoning. Hydrogenation was performed in a continuous stirred tank reactor keeping the catalyst confined inside the reactor with a closely woven wire net on the bottom. Observing
that deactivation was affected by water concentration and hydrogen pressure, we supposed that reversible poisoning occurs through the following reaction:
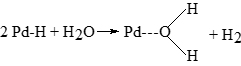
Reversible poisoning is responsible for an about 30% catalyst activity loss in 1 or 2 days of operation. Irreversible poisoning is much slower; to study it, we interpreted the activity data of an industrial plant simulating them for a 30 daysof continuous operation. It is interesting to observe that during the 30 days industrial run, fresh catalyst must be added in order to keep the catalytic activity constant up to about 200% of the initial amount. The necessity to add fresh catalyst to keep the catalytic activity in the industrial reactor constant is the most relevant consequence of catalyst poisoning. Similarly, we need to add EAQ to keep the optimal ratio EAQ/THEAQ constant, which is altered by the slow hydrogenation process for the aromatic rings of EAQ and THEAQ. These reactions do not affect the kinetic studies performed in laboratory reactors for the hydrogenation of anthraquinones to oxygen, but cannot be neglected in the simulation of big-sized continuous industrial plants
for long-time operations. Ring hydrogenation reactions are:
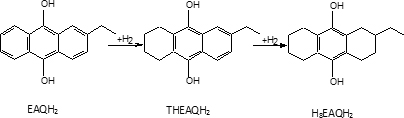
H8EAQH2 is inactive to produce H2O2. Other secondary reactions occur giving anthrones and dianthrones and a simplified reaction scheme can be proposed of the type (Santacesaria et al., 1984):
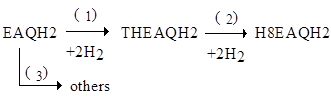
By observing experimental kinetic runs (Santacesaria et al., 1984), the formation of H8EAQH2 is strongly inhibited by the presence of EAQ. This behaviour agrees with a dual site mechanism for the ring hydrogenation; the kinetic relations used to simulate the three reactions examined were:
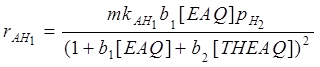
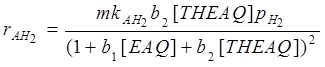
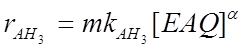
Problems involved in the second step: THEAQH2 oxidation
THEAQH2 oxidation is normally performed at industrial level in bubble column continuous reactors with air and with the working solution flowing countercurrent. Air flow rates are high enough to keep the liquid phase well mixed. The reaction occurs with a second order kinetic law of the type:
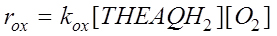
The kinetics has been studied using two different types of reactors such as: a continuous Levenspiel type reactor (Levenspiel and Godfrey, 1974), with a variable liquid interface obtained using floatings to avoid the formation of foams, and a laboratory semibatch gas-liquid reactor operating at variable stirring rates to produce a high gas-liquid interphase area. The Levenspiel reactor allowed to classify the reaction as moderately slow, that is, the enhancement factor is equal to about 1. In this condition, the oxygen mass transfer and reaction can be considered as consecutive steps and experimental data can easily be interpreted. The kinetic parameters obtained were:
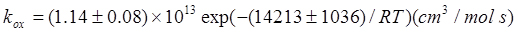
The mass transfer and oxygen solubility parameters were determined independently. The kinetic law and related parameters have successfully been tested simulating the bubble column of both a pilot and different industrial plants, the values of the gas-liquid interphase area in the examined bubble columns were somewhat uncertain as the working solution tended to give copious foam at the top of the reactor.
From the experimental viewpoint, it is interesting to observe that in the runs performed in the laboratory semibatch reactor no induction time has been observed, that is, steady state conditions for the involved radicals are readily achieved. Moreover, a second order kinetic law of the type previously seen requires a particular reaction mechanism in which the hydrogen bond is directly transferred to the oxygen molecule.
Problems involved in the fourth step: regeneration of active quinones and purification of the working solution
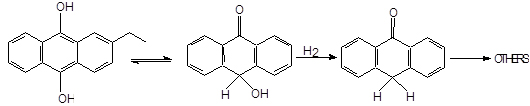
We have seen that during the hydrogenation step, secondary products are formed owing to the hydrogenation of aromatic rings. Normally, THEAQH2 is obtained from EAQ and THEAQH2 forms H8EAQH2. By analysis we have also seen small amounts of partially hydrogenated intermediates such as 2-ethylhexahydroanthraquinone H6EAQH2 and 2-ethyldihydroanthraquinone H2EAQH2. EAQH2 and THEAQH2 can also give tautomerization to oxanthrone followed by the formation of anthrone and dianthrone;
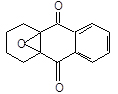
By - products can also be formed in the oxidation step, mainly epoxides of the type (Ulmann’s, 1994):
All these secondary products are formed as a consequence of very slow reactions not affecting the laboratory kinetic studies but they accumulate in the big-sized industrial plants and their reaction gives place to less soluble condensation products probably responsible for the permanent poisoning of the palladium-supported catalyst. Besides adding fresh EAQ to compensate the loss of active quinones, we therefore need to regenerate deteriorated molecules as much as possible and to purify the working solution from the formed tars and the acidity developed during the autoxidation step. Alkali supported on alumina or on silica-alumina with high surface area are normally used as catalysts and adsorbents in this reconversion step (Ulmann’s, 1994). The operation can be done on both the oxidized and reduced working solutions by feeding one of the mentioned solutions at about 100-150°C on a packed bed tubular reactor. The main reactions promoted by the alkaline catalyst are:
1. THEAQ dehydrogenation, mainly occurring in the oxidized solution with the following stoichiometry:
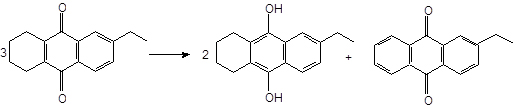
2. Epoxide reconversion, mainly occurring in a reduced solution with the following stoichiometry:
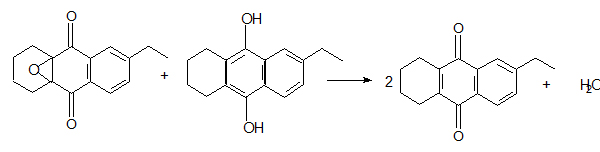
Other minor components can be reconverted to active quinones, for example H8EAQ can give THEAQ with a reaction similar to the previous one. Other convertible not identified quinones contribute to the reconversion, but the stoichiometry is not known and the contribution of each substance is very small, while the overall effect of these minor components is not negligible. So, we can consider reactions of the type:
3. Other convertible products  active quinones
active quinones
Apart from promoting the mentioned reactions, the alkaline catalyst neutralizes the free acidity of the working solution and induces the hydrolysis of 2-methylcycloacetate (one of the solvents) giving acetic acid and the corresponding alcohol. The acetic acid formed in this way contributes to poisoning catalyst by neutralization. At last, tar is adsorbed preferentially on the support with respect to the active quinones, the evaluated selectivity being:
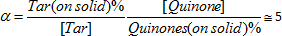
However, the most adsorbed active anthraquinone was THEAQH2 owing to the possibility of giving hydrogen bonds with the oxygens and hydroxyls of the solid surface. Exhausted alumina catalysts contain about 12% of organic compounds but only 2.4% of them are active quinones. Therefore, alkaline alumina catalysts have the effect of purging and regenerating the working solution developing three fundamental actions:
-
promotion of the three mentioned regenerating reactions
-
neutralization of the free acidity of the solution
-
depuration of the working solution by adsorbing preferentially tar.
A secondary negative effect is the promotion of the hydrolysis of one of the solvent components with a consequent autopoisoning effect. All the occurring phenomena have been studied separately at laboratory level to identify the kinetic and equilibrium expressions which will be used in a mathematical model for simulating the reconversion industrial section.
We studied in particular the THEAQ dehydrogenation and epoxide reconversion.
Kinetic runs of THEAQ dehydrogenation have been performed at 100 and 140°C in batch conditions by withdrawing and analysing small samples of the reacting mixture at different times. Reactions have been performed using 100 cm3 of aromatic hydrocarbons (C9-C12) in which 2g of THEAQ were dissolved. The catalyst was alumina (250 m2/g) containing about 4% of supported NaOH. The kinetics of the two mentioned reactions have been studied and related kinetic parameters determined.
-
E. Santacesaria, S. Carrà , R. Ferro; "The kinetics of the hydrogen peroxide industrial production". VIII CHISA, International Congress of Chemical Engineering, 3- 7/9/1984, Praga, Cecoslovacchia
-
E. Santacesaria, P. Wilkinson, P. Babini, S. Carrà; "Hydrogenation of 2-ethyltetrahydroanthraquinone in the presence of palladium catalyst". Ind. Eng. Chem. Res. 27, 780 (1988).

-
E. Santacesaria, M. Di Serio, R. Velotti, U. Leone; "Kinetics,Mass Transfer,and Palladium Catalyst Deactivation in the Hydrogenation Step of the Hydrogen Peroxide Synthesis via Anthraquinone." Ind. Eng. Chem. Research 33,2,277(1994)

-
E. Santacesaria, M. Di Serio, R. Velotti, U. Leone; “Deactivation of Palladium Catalyst in the hydrogenation of 2-ethyl 5,6,7,8-tetrahydroanthraquinone.” ( D. Delmon and G.F. Froment Eds.) Studies in Surface Science and Catalysis, Vol. 88, 597-602, Elsevier Science B.V. (1994).

-
E. Santacesaria, M. Di Serio, P. Iengo; “Examples of Hydrogenation in Semibatch and Continuous Slurry Reactors”. Catalysis Today 52 ,363-376 (1999).

-
E. Santacesaria, R. Ferro, S. Ricci, S. Carrà; "Kinetic aspects in the oxidation of hydrogenated 2- ethyltetrahydroanthraquinone". Ind. Eng. Chem. Res. 26, 155 (1987).

-
E. Santacesaria, M. Di Serio, A. Russo, U. Leone, R. Velotti; “Kinetic and catalytic aspects in the hydrogen peroxide production via anthraquinone”. Chem. Eng. Sci. 54, 2799-2806 (1999).

-
E. Santacesaria, M. Di Serio, R. Velotti, U. Leone; “Hydrogenation of the aromatic rings of 2-ethylanthraquinone on palladium catalyst”; J. of Molecular Catalysis 94, 37-46, (1994);
