Innovation in the production of ethyl acetate, acetic aldheyde and acetic acid
Summary of the work made
A new process, based on the use of a commercial catalyst for promoting the ethanol dehydrogenation to ethyl acetate, in one step reaction has been developed. A conversion of 65% with a selectivity to ethyl acetate of 98-99% has been obtained in relatively mild conditions.
Work
In the recent years, the interest in the ethanol production from renewable natural sources, as a possible alternative energy vector, has strongly grown in the world. The low-cost ethanol availability has also favored the study of the production of different chemicals such as ethylene, ethyl ether, acetaldehyde and ethyl acetate starting from ethanol as raw material. Ethyl acetate can be obtained directly from ethanol by dehydrogenation, today in a competitive way with respect to other previous processes considering the growing production of bio-ethanol for the fuel market. The direct conversion of ethanol to ethyl acetate, occurs according to the following reaction:
2C2H5OH → CH3COOC2H5+2H2
We have developed a new process based on the use of a commercial catalyst for promoting the ethanol dehydrogenation to ethyl acetate, in one step reaction. The best results have been found by using a commercial copper/copper chromite catalyst, supported on alumina and containing barium chromite as promoter, operating at 220-240°C, 20 bars and 98 (grams hour/mol) of ethanol contact time. In these conditions, a conversion of 65% with a selectivity to ethyl acetate of 98-99% has been obtained. However, the effect of temperature, pressure and ethanol contact time on both conversion and selectivity to ethyl acetate has been investigated. The catalyst has shown a good stability to deactivation. The behavior of this catalyst has been subject to a deeper investigation [1]. A patent has been published describing in detail the process [2]. A kinetic approach furnishes the information useful for reactor modeling [3]. It is
worth mentioning that this process has the advantage to produce pure hydrogen (exempt of CO) as by-product and very suitable for feeding fuel cells [1,2]. Moreover, it has been observed that at lower pressure (1-3 bars) acetic aldheyde is the main product. This opens the possibility to produce from ethanol all the acetic acid derivatives, that is, ethyl acetate and acetic aldheyde simply by changing the operative conditions, acetic acid by ethyl acetate hydrolysis and acetic anhydride with the current technology [4].
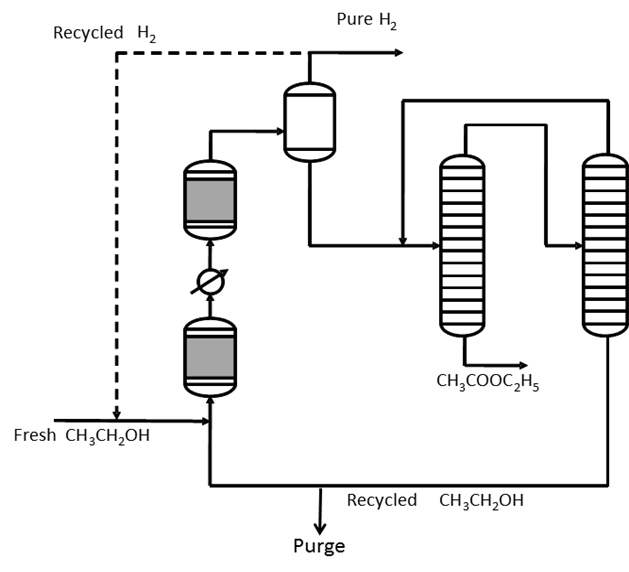
A simplified scheme of the process based on the use of a new copper/copper chromite commercial catalyst
Literature on ethanol dehydrogenation to ethyl acetate
-
E. Santacesaria, G. Carotenuto, R. Tesser, M. Di Serio; Ethanol Dehydrogenation to Ethyl Acetate by Using Copper and Copper Chromite Catalysts; Chemical Engineering Journal 179(2012)209-220.

-
G. Carotenuto, M. Di Serio, E. Santacesaria, R. Tesser; New process for the production of ethylacetate and and pure hydrogen from ethanol: Italian Patent NA2010A000009, 2010; WO2011/104738A2 assigned to EUROCHEM Engineering.
-
G. Carotenuto, R. Tesser, M. Di Serio, E. Santacesaria; Kinetic study of ethanol dehydrogenation to ethyl acetate promoted by a copper and copper chromite catalyst; Catalysis Today: 203(2013)202-210.

-
G. Carotenuto, R. Tesser, M. Di Serio, E. Santacesaria; Bioethanol as feedstock for chemicals such as acetaldheyde, ethyl acetate and pure hydrogen; Biomass Conv. Bioref.(2013) vol. 3 Issue 1:55-67
