Epoxidation of soybean oil to obtain ESBO
Summary of the work made
The reaction of soybean or other unsatured oils with hydrogen peroxide, in the presence of formic or acetic acid as oxygen transfers, and of a mineral acid as catalyst, is an extremely exothermic reaction. Suitable operative conditions must be adopted for working in safe conditions. We have studied in detail what are the best operative conditions. Moreover, a new technology is under development using a solid acid catalyst that greatly simplify the process, reducing the costs and increasing the productivity.
Work
Epoxidized oils are important chemicals, normally used as plasticizers and stabilizers for PVC resins. Nowadays, it is very important to increase the epoxidized soybean oil (ESBO) productivity, because, this substance is a good substitute of phtalates, as plasticizer, since phthalates have been banned in many countries for their negative effects on health. The epoxidation of vegetable oils, for example soybean oil, is normally carried out by reacting the double bonds of the oil with a peroxyacid (generally peroxyacetic or peroxyformic acid) generated in situ by reacting concentrated hydrogen peroxide with acetic or formic acid in the presence of a mineral acid as catalyst. The peroxyacid formation occurs in the aqueous phase according to the following reaction:

Then, performic acid migrates into the oil phase and reacts spontaneously according to the following scheme:
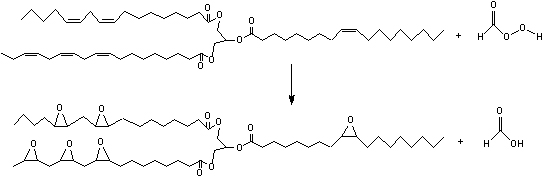
This reaction is highly exothermic (ΔH= -55 Kcal/mol for each double bond) and an excessive increase of the temperature, in the reactors is prevented by adding a limited amount of a mixture of H2O2 and formic or acetic acid to the mixture of oil and acid catalyst. The reaction, normally, requires 8-10 hours to be completed keeping the temperature between 60 and 75°C. An optimization of this process requires detailed information about both the kinetics of the occurring reactions and the effects of heat and mass transfer [1,4]. These aspects have been studied in details by our research group and we are now ready to model and build a pilot plant using the described conventional technology. In the meantime, we are developing new technologies based on the use of solid acid catalyst [5,6] instead of soluble mineral acids (sulphuric, phosphoric acids). This work has been made in collaboration with MYTHEN SpA, that is, an ESBO producer.
Soybean oil epoxidation in the presence of mineral acids as catalyst
-
E. Santacesaria; R. Tesser; M. Di Serio; R. Turco; V. Russo; D. Verde; A biphasic model describing soybean oil epoxidation with H2O2 in a fed-batch reactor; Chemical Engineering Journal (2011), 173(1), 198-209.

-
D. Kralisch, I. Streckmann, U. Krtschil, E. Santacesaria, M. Di Serio, V. Russo, L. DeCarlo, W. Linhart, E. Christian, B. Cortese, M.H.J.M. de Croon, V. Hessel; Transfer of the Epoxidation of Soybean Oil from Batch to Flow Chemistry – Guided by Cost and Environmental Issues; ChemSusChem; 5(2012)300-311.

-
E. Santacesaria, A. Renken, V. Russo, R. Turco, R. Tesser, M. Di Serio; Biphasic Model Describing Soybean Oil Epoxidation with H2O2 in Continuous Reactors; Industrial & Engineering Chemistry Research 51 (2012) 8760-8767.

-
E. Salzano, A. Garcia Agreda, V. Russo, M. Di Serio; Safety Criteria for the Epoxydation of Soybean Oil in Fed-Batch Reactor; E. Santacesaria; Chemical Engineering Transaction 26 (2012) 39-44.

Soybean oil epoxidation in the presence of solid acids as catalyst
-
M. Di Serio, R. Turco, P. Pernice, A. Aronne, F. Sannino, E. Santacesaria; Valuation of Nb2O5-SiO2 catalysts in soybean oil epoxidation; Catalysis Today: 192 (2012) (1), 112-116

-
R. Turco, R. Vitiello, V. Russo, R. Tesser, E. Santacesaria, M Di Serio; Selective epoxidation of soybean oil with performic acid catalyzed by acidic ionic exchange resins; Green Processing and Synthesis 2 (5), 427-434 (2013)
