Baker yeast production optimization
Summary of the work made
A cybernetic highly sophisticated model has been developed and successfully applied to both fed batch and continuous bioreactors of different sizes from laboratory to industrial size bioreactor in order to predict the baker yeast production. By using this model, in a predictive way, it is possible to optimize the feeding flow rates of nutrients and oxygen reaching the optimal yield of yeast. Moreover, oxygen can be dosed by using membrane tubes of opportune length.
Work
Saccharomyces cerevisiae biomass in the form of baker's yeast, represents the largest bulk production of any single-cell microorganism in the world. Several million tons of fresh baker's yeast cells are produced yearly for human food use. The production of baker's yeast involves the multi-stage propagation of the selected yeast strain on sugar as a carbon source. Baker’s yeast is usually produced starting from a small amount of yeast added to a liquid solution of essential nutrients (molasses, ammonia or ammonium salts, phosphate and vitamins), at a suitable temperature and pH. Once the cell population has grown enough, it is transferred into a larger bioreactor for a new growth stage; 4 or 5 stages are usually necessary to reach a satisfactory production quantity. Therefore, the bioreactor volume changes from 1÷10 dm3 to 100÷150 m3. The smaller bioreactors used for the initial stages operate in batch and anaerobic conditions, whereas, in the larger
bioreactors used for the later stages, aeration is provided and the fed-batch cultivation mode is adopted, i.e., the nutrients are fed to the culture medium at a variable rate. The plant configuration and operative choices are the consequence of the effects that S. cerevisiae metabolism has on biomass yield and growth rate. During the aerobic growth of S. cerevisiae, both sugars and ethanol can be used as carbon and energy sources. Sugars can be metabolised via two different energy producing pathways -fermentation or oxidation- depending on the sugar concentration in the medium. Indeed, at a high sugar concentration, oxidation is suppressed and fermentation takes place (the Crabtree effect); oxidation predominates when sugar concentration is below 50-100 mg/dm3. On the other hand, under oxygen-limited growth conditions, the fermentative pathway leading to ethanol production predominates, even at a low sugar concentration. During aerobic growth on sugars, the ethanol produced during
the initial fermentative metabolic pathway is consumed when sugars are no longer available in the medium. Biomass yields on sugars (gcells/gsugars) are strongly related to the prevailing metabolic pathway, being maximal only when sugar is oxidised, i.e. when its concentration remains below 0.05-0.1 g/dm3. Contrarily, volumetric biomass productivity (gcells/dm3 h) is maximal if the specific growth rate is maximal, i.e. at a high sugar concentration, when the fermentative pathway is operative.
In the first stages of industrial production, a batch cultivation mode is employed, thus favouring productivity instead of biomass yield, since the total biomass produced in small reactors is low. In the following stages carried out in larger reactors, it is necessary to optimise both yield and productivity, and a fed-batch cultivation mode is employed. Therefore, the initial feed profile is selected to give a high growth rate when total biomass is still low (low yield/high productivity); under these conditions some ethanol is produced. Later, the feed profile is fixed in such a way as to favour oxidative metabolism (high yield/low productivity), and the ethanol initially formed is also consumed.
The control of the molasses feed profile, performed with a feedback loop based on data collected from exhaust gas analysis (O2 and CO2) and/or ethanol sensors, has not proved to be economically advantageous, so industrial fed-batch production of baker’s yeast is still carried out in open loop conditions, and the empirically established molasses feed profiles are kept as manufacturing secrets. Other than feed control, optimisation of the entire bioprocess through the choice of optimal bioreactor size, process duration, and initial concentration of inoculum is the goal of industrial production. Major problems arise from the fact that yeast growth rate depends on both the strain employed and composition of molasses, the latter varying considerably according to the refinery technology, agricultural and climate conditions. This limits the use of an expert program for optimisation which requires that the information acquired in previous runs should be stored in the
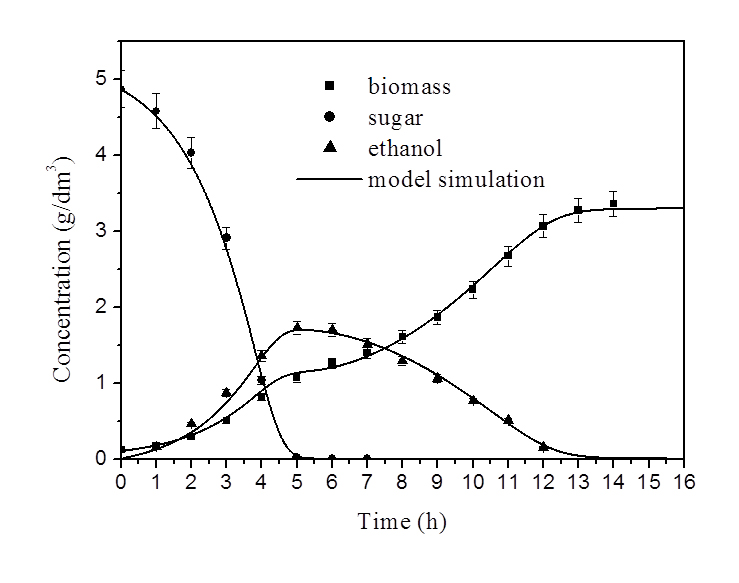
system data base and used as input for programming the successive nutrient fed operation. We showed that a cybernetic approach [1-3] is suitable for the simulation of baker’s yeast growth in both laboratory and industrial bioreactors and we have investigated the effects that factors such as type of strain, culture medium and physiological state of the inoculum exert on yeast growth, by carrying out experimental batch and fed-batch runs. The quantitative analysis of the obtained data was performed by means the previously mentioned cybernetic model. A sensitivity analysis of the model’s kinetic parameters was also performed to establish the parameters that could be determined by mathematical regression on the experimental data. This model has been verified by simulating fed-batch growth runs, carried out in operative conditions different from those used to determine the model’s kinetic parameters, so demonstrating its predictive power. The model has been successfully applied to both fed
batch and continuous bioreactors of different sizes from laboratory to industrial size bioreactor. By using the model in a predictive way it is possible to optimize the feeding flow rates of nutrients and oxygen. This last could be dosed by using membrane tubes of opportune length [4]. This work has been conducted in collaboration with Pressindustria SpA.
-
M. Di Serio, P. Aramo, E. de Alteriis, R. Tesser, E. Santacesaria; “Quantitative analysis of the key factors affecting yeast growth”; Ind. Eng. Chem. Research (2003), 42, 5109-5116

-
M. Di Serio, R. Tesser, E. Santacesaria;“A kinetic and mass transfer model to simulate the growth of baker’s yeast in industrial bioreactors”. A Special Issue on “Frontiers in Chemical Reaction Engineering” Chem. Eng. Journal 82(2001) 347-354.

-
M. Di Serio, E. De Alteris, P. Parascandola, E. Santacesaria;“A general kinetic and mass transfer model to simulate the baker’s yeast growth in bioreactors”. Catalysis Today 66 (2001) 437-445.

-
Tesser, Riccardo; Bottino, Aldo; Capannelli, Gustavo; Montagnaro, Fabio; Vitolo, Stefano; Di Serio, Martino; Santacesaria, Elio; “Advantages in the Use of Membrane Contactors for the Study of Gas-Liquid and Gas-Liquid-Solid Reactions.” Industrial & Engineering Chemistry Research (2005), 44(25), 9451-9460